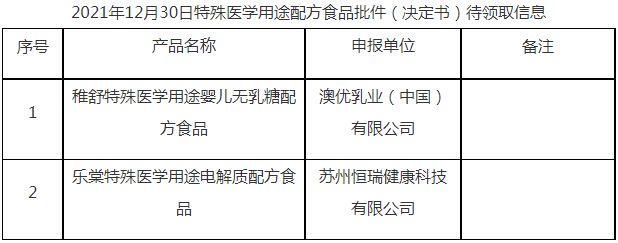
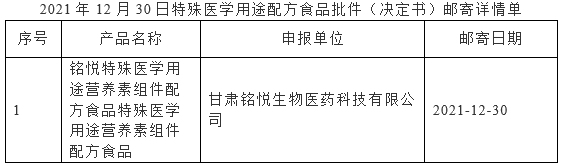
On December 30, 2021, the Center of Food evaluation of the State Administration for Market Regulation updated the list of approval document (decision) for the registration of infant formula milk powder product formula and formula food for special medical purpose (FSMP), which includes 12 kinds of infant formula products such as Jiyou older infant formula milk powder (6-12 months old, stage 2), Yabeile infant formula milk powder (0-6 months old, stage 1), and 3 FSMP products such as Zhishu special medical use infant lactose-free formula food.
国家市场监督管理总局食品审评中心发布2021年12月30日婴幼儿配方乳粉产品配方注册批件(决定书)邮寄详情单,涉及吉优较大婴儿配方奶粉(6—12月龄,2段)、芽倍乐婴儿配方奶粉(0—6月龄,1段)等12种婴幼儿配方乳粉产品配方注册批件(决定书)待领取信息,稚舒特殊医学用途婴儿无乳糖配方食品等3种特殊医学用途配方食品批件(决定书)邮寄详情单。